Role of Hyperbaric Oxygen Therapy in Renal Regeneration in End Stage Renal Disease and Hemodialysis
Renal Disorders
The kidneys are two organs that resemble beans. Your kidneys produce urine while removing surplus water and waste from your blood. Your kidneys are harmed & unable to filter blood properly if you have renal disease. Due to the lack of available organ donors and therapies, renal disease and illness are global public health concerns. When kidney structures, such as nephrons, are unable to perform their duties, illnesses, and diseases like these develop.
A startling one in every three American people is at risk for developing kidney disease, making it one of the most prevalent ailments. This condition has the potential to progress to end-stage renal disease (ESRD), also known as kidney failure and characterized by the total loss of renal function. Malignant hypertension, hyperglycemia, glomerular, tubular, and interstitial disorders are additional prevalent conditions [1]. Dialysis or a kidney transplant has historically been used to treat renal illnesses like ESRD. But there is a disparity between the number of patients who need therapy and the readily available medicines. Over 89,000 people die annually because of inadequate dialysis and a lack of transplantable kidneys. A fresh possibility in this horrifying reality is made apparent by the prospect of kidney tissue regeneration.
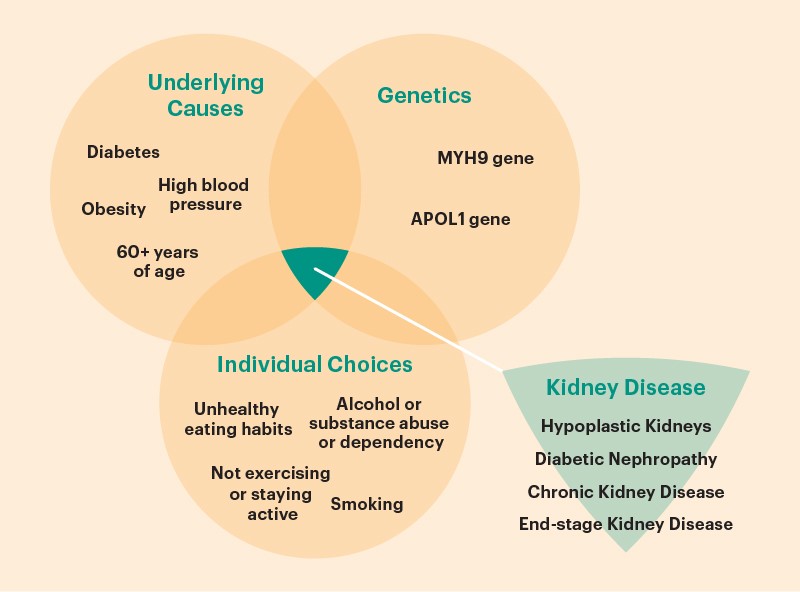
Symptoms
Early warning indications of potential renal disease include the symptoms listed below:
- trouble sleeping
- loss of appetite
- cramps in the muscles
- swelling in the ankles and feet
- excessive urination, especially late at night
Significant indicators indicating kidney failure is developing because of your kidney illness include:
- nausea
- vomiting
- reduced appetite
- shifts in urine production
- anemia and fluid retention
- hyperkalemia
- Pericardial inflammation

Renal Regeneration
Kidney regeneration enables the regrowth and repair of damaged renal structures, which restores function. Since nephrons originate during embryonic development, adult regeneration is not conceivable [2]. Animal models help us to comprehend neon progenesis, a process that culminates in the development of new nephrons. Human pluripotent stem cell research on kidney morphogenesis and identification of these animal models have improved the development of kidney cells. Both human and animal models are now being used to study stem cells [3]. If you have diabetes or high blood pressure, you are more likely to develop renal disease. Dialysis or a kidney transplant are available as therapies for renal failure. Acute renal damage, kidney cysts, kidney stones, and kidney infections are a few other kidney issues.
End Stage Renal Disease
End-Stage Renal Disease (ESRD) is a medical illness in which a person’s kidneys stop working permanently, necessitating either a kidney transplant or a continuous course of long-term dialysis to sustain life. A type of renal replacement therapy is dialysis. Artificial machinery is used to augment the kidney’s function of filtering the blood to remove extra water, solutes, and poisons. When a person has acute kidney injury (AKI), which is characterized by a sudden loss of kidney function, or chronic kidney disease, which is characterized by a slow, progressive loss of renal function, dialysis ensures the maintenance of homeostasis (a stable internal environment) (CKD, previously end-stage renal disease, ESRD). In 2010, over 2.5 million individuals got chronic renal replacement treatment (RRT). It is the cornerstone of the care of end-stage renal disease, a kidney ailment with an increasing global burden mostly caused by diabetes mellitus (45%) and hypertension (30%). Hemodialysis, peritoneal dialysis, & hemofiltration are the three main forms of dialysis [4].
Hemodialysis
Hemodialysis involves pumping blood through a specialized device that removes waste materials and fluid. Hemodialysis can be conducted at home, in a hospital, or at a dialysis facility. The average person attends three sessions per week, each lasting between three and five hours. Hemodialysis can, however, also be performed more frequently and in shorter periods.
Most patients will undergo surgery to develop an arteriovenous (AV) fistula a few weeks before beginning hemodialysis. An artery and a vein are connected beneath the skin, usually in the forearm, to form an AV fistula. During hemodialysis treatment, the bigger blood artery enables a continuous flow of more blood across the body. This suggests that more blood can be cleaned and filtered. If an artery and vein cannot be connected, an arteriovenous graft (a looped, plastic tube) may be implanted and used for the same function. Low blood pressure, cramps, and itching are the most frequent hemodialysis adverse effects.

Hyperbaric Oxygen is Effective Medicine for Renal Regeneration in End Stage Renal Disease and Hemodialysis
By administering pure oxygen under high pressure, hyperbaric oxygen treatment (HBOT) increases the amount of oxygen in the blood and tissues (hyperoxia) (about 2-3 atmospheres). One kind of treatment involves exposing patients to pure oxygen (O2) concentrations at high atmospheric pressures, which is known as hyperbaric oxygen therapy (HBOT). As per the Undersea and Hyperbaric Medical Society, this pressure may be greater than or equivalent to 1.4 atmospheres. For all current UHMS-approved usage, patients may only use oxygen when restrained in an environment with a minimum air pressure of 2 ATA. Hyperbaric medicine requires a pressure environment that is greater than the air pressure at sea level.
A 2015 study by Verma et al. explored the possibility that HBOT would lessen the kidney damage caused by diabetes by utilizing db/db mice, an obese, leptin deficient T2DM rodent model. This study investigated several different urinary biomarkers (NAC, NGAL, KIM1, CyC), which showed that 20 weeks of HBOT had a cytoprotective impact on renal tissue. HBOT specifically decreased the amounts of CyC in the urine, the biomarker most strongly associated with tubule function and a measure of glomerular filtration rate. This shows that tissue damage has been suppressed in both the proximal convoluted tubules and glomeruli, maintaining kidney function as a result. Also discovered to be a particularly sensitive responder to HBOT is neutrophil gelatinase-associated lipocalin (NGAL), a renal marker of harm from ischemia and inflammation. Following HBOT, NGAL levels reduced, indicating a repair of the DKD-related early damage in this mouse model. When compared to untreated controls, HBOT at 2.4 ATA dramatically lowered the albumin to creatinine ratio in db/db mice, which is consistent with lessened glomerular membrane damage. These findings were thought to be in line with the protection of the glomerular filtration membrane in microvascular vessels [5].
Increased glucose levels in db/db mice cause the mitochondria, ER, and oxygen-consuming NADPH oxidase family enzymes to produce too much ROS. These ROS is responsible for the development of persistent inflammation, which can result in end-stage renal failure (ESRD). It is interesting to note that both hypoxia and HBOT cause an increase in ROS, which contributes to the pathophysiology of DKD and the development of ESRD in some cases. To resolve this issue, Verma et al. (2015) propose that HBOT introduces oxygen-saturated blood into tissues, restoring normal oxygen levels and lowering endogenous, pathologic ROS generation. This may be achieved by limiting the overloading of electron carriers in the mitochondrial electron transport chain. By restoring normal oxygen levels in tissues and lowering the demand placed on electron carriers through tissue (mitochondrial) oxygenation, HBOT helps to minimize the generation of endogenous ROS and the quantity of pathologic ROS. HBOT enhances mitochondrial function, which in turn restores tissue bioenergetics to aid in healing by allowing oxygen to permeate damaged and diseased tissues [6].
Calciphylaxis is a syndrome of tiny artery calcification with an uncertain cause that results in painful violaceous skin lesions that proceed to non-healing ulcers and gangrene. It is also said calcific uremic arteriolopathy. It is primarily seen in ESRD patients, with a reported frequency of 1-4% in those on chronic haemodialysis. Five patients were

given HBOT, two of whom were receiving chronic haemodialysis and three of whom were receiving continuous ambulatory peritoneal dialysis (CAPD). Each patient received 25 to 35 doses of HBO2 at 2.5 atmospheres, each lasting 90 minutes. In conclusion, HBO2 treatment resulted in the full healing of the calciphylaxis lesions in two of the study’s five participants. Most calciphylaxis patients do not have uncontrolled hyperparathyroidism, which limits their treatment options. These findings imply that HBOT is risk-free and that it can be used to treat calciphylaxis [7].
References
1. Lin, Y. Q., Wang, L. R., Pan, L. L., Wang, H., Zhu, G. Q., Liu, W. Y., … & Zheng, M. H. (2016). Kidney bioengineering in regenerative medicine: An emerging therapy for kidney disease. Cytotherapy, 18(2), 186-197.
Link: https://www.sciencedirect.com/science/article/pii/S1465324915010786
2. Chou, Y. H., Pan, S. Y., Yang, C. H., & Lin, S. L. (2014). Stem cells and kidney regeneration. Journal of the Formosan Medical Association, 113(4), 201-209.
Link: https://www.sciencedirect.com/science/article/pii/S0929664613004300
3. Harari-Steinberg, O., Pleniceanu, O., & Dekel, B. (2011). Selecting the optimal cell for kidney regeneration: fetal, adult, or reprogrammed stem cells. Organogenesis, 7(2), 123-134.
Link: https://www.tandfonline.com/doi/full/10.4161/org.7.2.15783
4. Lameire N, Van Biesen W. The initiation of renal-replacement therapy–just-in-time delivery. N Engl J Med. 2010 Aug 12;363(7):678-80. doi: 10.1056/NEJMe1006669. Epub 2010 Jun 27. PMID: 20581421.
Link: https://pubmed.ncbi.nlm.nih.gov/20581421/
5. Verma, R., Chopra, A., Giardina, C., Sabbisetti, V., Smyth, J. A., Hightower, L. E., & Perdrizet, G. A. (2015). Hyperbaric oxygen therapy (HBOT) suppresses biomarkers of cell stress and kidney injury in diabetic mice. Cell Stress and Chaperones, 20(3), 495-505.
Link: https://link.springer.com/article/10.1007/s12192-015-0574-3
6. Zhou, Q., Huang, G., Yu, X., & Xu, W. (2018). A novel approach to estimate ROS origination by hyperbaric oxygen exposure targeted probes and specific inhibitors. Cellular Physiology and Biochemistry, 47(5), 1800-1808.
Link: https://www.karger.com/Article/Abstract/491061
7. Tiina Podymow, Chris Wherrett, Kevin D. Burns, Hyperbaric oxygen in the treatment of calciphylaxis: a case series, Nephrology Dialysis Transplantation, Volume 16, Issue 11, November 2001, Pages 2176–2180, https://doi.org/10.1093/ndt/16.11.2176
Link: https://academic.oup.com/ndt/article/16/11/2176/1933020

